Join the registry to be counted no matter where you are in the world, help advance the understanding of Leigh syndrome, raise awareness for the need for treatments, and stay informed on research and clinical trial opportunities.
Watch video
Mia, USA
Leigh Syndrome Global Patient Registry
If you or a family member has been diagnosed anywhere in the world with Leigh Syndrome, please enroll in the patient registry to make an important contribution to Leigh Syndrome research.
60%
diagnosed within 1 year from symptoms onset
2 years old
median age when diagnosed
1-31
Number of specialists seen
The Cure Mito Foundation is partnering with Sanford CoRDS to create the registry. Sanford CoRDS supports and enables rare disease communities to build robust registries, providing researchers with the information they need to drive research forward.
Enrollment Instructions
If you would like to enroll and complete the CoRDS Patient Registry Questionnaire online, you may do so by following the instructions below. The registry works best with updated versions of Google Chrome, Mozilla Firefox or Microsoft Edge and may not work as well with a phone or tablet. You may also complete registration on paper by calling CoRDS at + 1 877 658 9192 or sending an email to cords@sanfordhealth.org and requesting a paper version of the CoRDS Patient Registry Questionnaire.
Watch video
Important
Prior to registering, please create your Clininical Research ID (CRID). Creating a CRID takes less than 2 minutes .To obtain a CRID please visit www.thecrid.org. You will be asked to enter your CRID in the last question on the registry survey.
”Cure Mito Foundation’s Leigh syndrome global patient registry is foundational – it will catalyze basic research while also helping to get medicines approved by the regulatory agencies.
Dr. Vamsi Mootha, MDHarvard Medical School, Massachusetts General Hospital, Broad Institute of MIT and Harvard
Important Registry Reminders and Info
-
Please obtain your Clinical Research ID (CRID) by visiting www.thecrid.org. You will be asked to enter your CRID in the last question on the registry survey.
-
After completing or updating a survey, it is important to hit “Submit”. Otherwise your data will not be available for analysis.
-
Registry has a general survey and a Leigh syndrome survey. Both should be completed for data to be included in the analysis.
-
If diagnosis has been confirmed by genetic testing, please enter genetic mutation in question 4 in the Leigh syndrome survey. If you need help interpreting your genetic report please contact us at info@curemito.org.
-
If your loved one turns 18, you will be sent an email from CoRDS asking you to re-consent. 3 reminders will be sent. If you don’t re-consent, your data will no longer be available
-
If you are enrolling a participant who has both Leigh syndrome and PDCD, during account creation it is important to select “Leigh Syndrome” and “Pyruvate Dehydrogenase Complex Deficiency (PDCD)” under “Rare disease diagnosis”.
-
If you have more than one family member with Leigh Syndrome – please enroll each participant separately. Your family’s participant accounts can later be linked by clicking “Family Branching” in the participant portal.
-
Surveys are available in Spanish, Portuguese, French, Italian, Turkish, Polish, Chinese. All translations are certified and IRB-approved. Please reach out to us at info@curemito.org if you need translated surveys. If you’re interested in other languages please reach out to us as well.
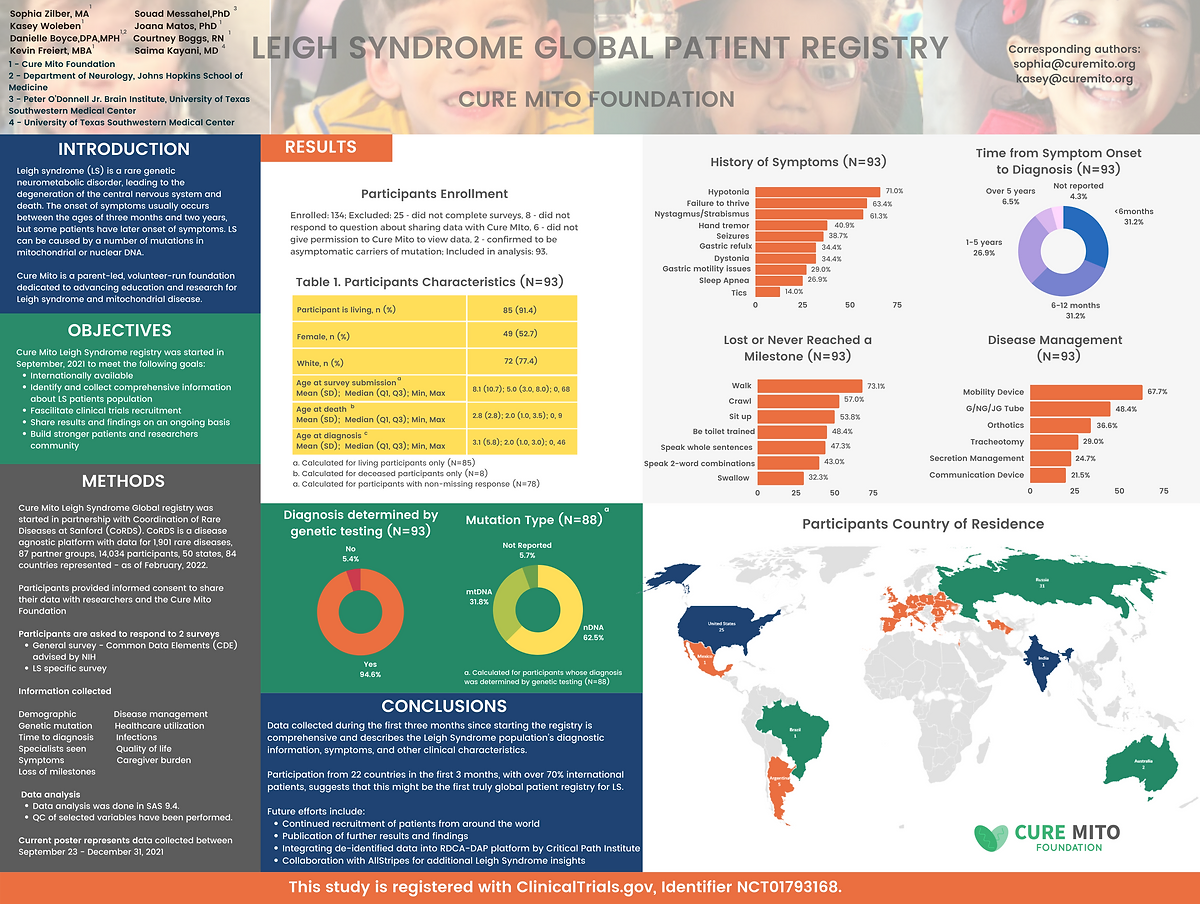
Registry Results
Interoperability of Leigh Syndrome Patient Registry Data with Regulatory Submission Standards
Parag Shiralkar, Bakare, P., Woleben, K., Zilber, S., Parag Shiralkar, Bakare, P., Woleben, K., & Zilber, S. (2024). Interoperability of Leigh Syndrome Patient Registry Data with Regulatory Submission Standards. Journal of the Society for Clinical Data Management, 4(1). https://doi.org/10.47912/jscdm.244
Read Full PaperLeigh Syndrome Global Patient Registry: Uniting Patients and Researchers Worldwide
Zilber, S., Woleben, K., Johnson, S. C., Moura de Souza, C. F., Boyce, D., Freiert, K., Boggs, C., Messahel, S., Burnworth, M. J., Afolabi, T. M., & Kayani, S. (2023). Leigh syndrome global patient registry: uniting patients and researchers worldwide. Orphanet Journal of Rare Diseases, 18, Article 264. https://doi.org/10.1186/s13023-023-02886-0
Read Full PaperStandardizing Leigh Syndrome Patient Registry Data to the OMOP Common Data Model by Using Open Source R
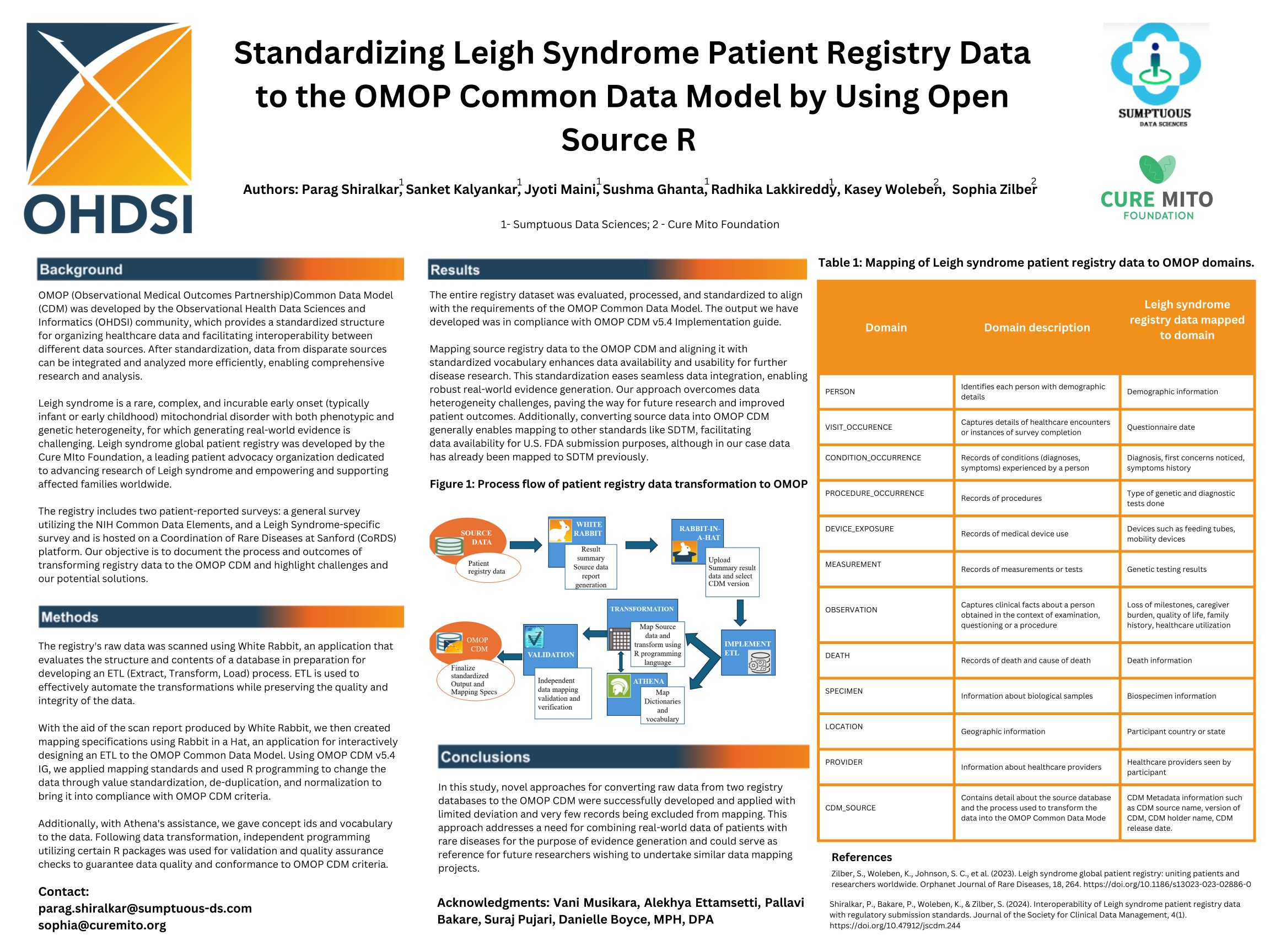
Alignment and Interoperability of Leigh Syndrome Registry Data with Regulatory Submission Standards
Registry Milestones
November 2021
Cure Mito collaborates with C-Path on data sharing into C-Path’s RDCA-DAP® platform. PRESS RELEASE.
September 2023
Our paper: “Leigh Syndrome Global Patient Registry: Uniting Patients and Researchers Worldwide” is published in the Orphanet Journal of Rare Diseases.
January 2024
Cure Mito launches Mitochondrial and Metabolic disease taskforce with C-Path and seven other stakeholders. PRESS RELEASE.
January 2024
Our paper: “Interoperability of Leigh Syndrome Patient Registry Data with Regulatory Submission Standards” is published in the Journal of the Society for Clinical Data Management.
Feburary 2024
Cure Mito collaborates with Hope for PDCD foundation to launch PDCD registry on the same platform in alignment with Leigh syndrome registry. PRESS RELEASE.
Frequently asked questions
What is a patient registry?
A registry is a program for collection, storage, retrieval, and dissemination of clearly defined information for a specific purpose. Data collected in this registry includes diagnosis and treatment, management of care, quality of life, and longitudinal information for Leigh Syndrome.
Why a Leigh Syndrome registry?
As researchers and patients with Leigh Syndrome are located all around the world, having a centralized database makes it easier for clinical information to be shared. The registry provides an excellent opportunity for families to be involved in research studies and clinical trials. Once questionnaires are completed, researchers can compare specific symptoms, identify how the syndrome progresses, as well as gain valuable insights into which treatments were most successful.
Who should participate?
Everyone with Leigh syndrome—including those who have passed away. Participating in the Registry at CoRDS is a great way for participants and their caregivers to take part in helping to identify the specific symptoms, and treatments of Leigh syndrome.
I don’t have a confirmed diagnosis. Should I still participate?
We recommend that anyone with a suspected diagnosis of Leigh Syndrome join the registry. If diagnosis is later confirmed, patients can update their genetic mutation and upload their genetic report.
I don't wish to participate in any research or clinical trials. Should I still register?
Even if you do not wish to participate your information will still be essential for researchers studying Leigh Syndrome. Just by registering you are making a difference; your participation helps the researchers gain a better understanding of Leigh Syndrome.
What information would I be asked to share?
Patients or caregivers of patients will be asked to fill out two surveys – a general survey and a Leigh Syndrome specific survey. If patients have a confirmed genetic diagnosis, they will also be asked to upload their genetic report. Having a genetic report is helpful as it allows us to verify a patient’s genetic mutation, which is critical in order to understand the disease and connect patients to the appropriate research or clinical trial opportunities.
How were the surveys developed?
General survey was provided by the CoRDS registry and is based on the survey developed by the National Institutes of Health (NIH). Disease specific survey was developed by Cure Mito in collaboration with our patient registry advisors and industry representatives and reviewed by our scientific advisory board members.
Do I have to respond to all questions in surveys?
You can skip questions, however, the more complete data there is, the more useful it will be for researchers. Therefore, we encourage everyone to respond to all questions. You can stop and come back at a later time.
How will my data be used by Cure Mito?
If you choose to share your data with Cure Mito, we will analyze the data and may get back to you if any responses are missing or need further clarification. This would be done in order to assure that data is as complete and accurate as possible, which is critical in order for it to be useful to researchers. We will summarize the findings and share with patients and researchers. We may also contact you occasionally to share additional research opportunities or online surveys that you can choose to participate in.If you have any questions or concerns about sharing your data, we encourage you to reach out to us at info@curemito.org.
Will results of my participation in the registry be shared with me?
Yes, all data that is shared with Cure Mito will be analyzed and results shared with the community in a timely manner.
Who will decide which researchers can access the data?
The de-identified data will be shared only with researchers approved by Sanford’s Scientific Advisory Board (SAB) together with representatives from Cure Mito. No personal identifying information (such as patient’s or caregiver’s name, emails, addresses) will be shared.
Can my participation help other rare diseases?
Yes! Because CoRDS uses exactly the same general survey for all rare diseases, data can be easily harmonized and collated based on a disease characteristic or symptom. These capabilities help researchers understand what causes rare diseases and develop treatments.
Is personal information safe?
Internet polls, questionnaires, and surveys are often used to collect information quickly and easily from respondents. However, to publish their results, researchers must only use information obtained in a specific way. The patient data they use must have written approval from patients to use their data this way and must adhere to strict privacy regulations. The Leigh Syndrome registry is protected by the Health Insurance Portability and Accountability Act (HIPAA) and compliant with the European Union General Data Protection Regulation (GDPR). CoRDS has worked hard to ensure that researchers can use the information you provide.
CoRDS submits every questionnaire to their Institutional Review Board for approval. The IRB is a group that reviews the ethics of medical research studies.
Informed consent also safeguards participant data. When registering to participate in CoRDS, participants (or their representatives) are given a chance to read the consent documentation before filling out the questionnaire. For any questions, participants can call CoRDS at + 1-877-658-9192 or send an email to cords@sanfordhealth.org.
If researchers receive approval to look at the registry or questionnaire responses, they will be given only the anonymous data without the identifiable information.
Your privacy is also protected even if you indicate that you are willing to be contacted for additional research. For example, a researcher might contact CoRDS to ask for additional information from all participants who have a specific symptom, such as loss of hearing. CoRDs would then contact every participant in the registry who said that they had loss of hearing and that they would like to participate in additional studies. CoRDS would then provide the participants with the researcher’s contact information, and it would be up to the individual participants to contact the researcher to participate in additional research. CoRDS will never provide your contact information to anyone.
When should I update registry information?
Updates can be done any time and should only take 10-15 minutes. But updates should be done at least every year, after any significant change in the participant’s health, and immediately after their 18th birthday. If the participant was enrolled in the registry as a minor, their data becomes inaccessible 30 days after their 18th birthday unless and until they are reenrolled and sign a consent form themselves.
Can data be collected worldwide?
Yes, the registry can be accessed all over the world with the link provided on our website. International participation is highly encouraged.
How is my personal information used and protected?
The information you provide will be provided to researchers studying Leigh Syndrome. CoRDS has put many safeguards into place to ensure that this information will be kept safe and confidential. The registry is protected by the Health Insurance Portability and Accountability Act (HIPAA) and compliant with the European Union General Data Protection Regulation (GDPR).
This FAQ has been approved by Sanford IRB. For any questions, participants can call CoRDS at + 1-877-658-9192 or send an email to cords@sanfordhealth.org.